
Sodium Hyaluronate (HA) is a polysaccharide that has wide use in the pharmaceutical, cosmetic, and food industries. Its quality will immediately impact the end products’ safety and efficiency. To ensure the quality of sodium hyaluronate, rigorous testing needs to be performed. Then, how to test sodium hyaluronate powder? Hereafter, we take our company’s testing process as an example:
1. Visual Inspection
1.1 Procedure
Wear gloves, and set a clean white sheet of paper at the bottom of a stainless steel tray. Set the 100g of the sodium hyaluronate sample on the tray and examine the color and texture of the powder in bright, indirect sunlight. Gently scrape the sample with a spoon or stainless steel ruler and examine for foreign particles or impurities. Remove them if they are present and dissolve in water; failure to dissolve in water indicates impurities.
1.2 Recording & Requirements
Record the color observed, the texture, the presence of foreign particles, and the number of impurities observed. The product must have no foreign particles, and no more than five impurities in each 100g of the sample may be tolerated.
2. Loss on Drying Test
2.1 Measurement
Replace the sample in a pre-dried and constant weight flat weighing bottle (W1) and weigh it precisely (W2). Dry the sample in an electric forced-air drying oven for 6 hours. When drying, do not have the bottle cap on, and leave the bottle half open or place it beside the weighing bottle. After drying, cover the bottle right away and place it in a desiccator, and let it cool to room temperature for weighing (W3).
2.2 Calculation
The formula for loss on drying (h%) is:
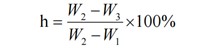
Where:
- W₂: Total weight of sample and weighing bottle before drying (g)
- W₃: Total weight of sample and weighing bottle after drying (g)
- W₁: Weight of the empty weighing bottle (g)
3. Molecular Weight & Intrinsic Viscosity Testing
The molecular weight determines HA’s rheological properties and applications. For example, low-molecular-weight HA is more easily absorbed, while high-molecular-weight HA is better for filling and moisturizing.
- Method: Use an Ubbelohde viscometer to measure solution flow time and calculate intrinsic viscosity and average molecular weight.
- Standard: Different HA applications require different molecular weight ranges, which must meet corresponding specifications.
4. pH Testing
pH affects HA’s solubility and biocompatibility.
- Method: Dissolve the sample and measure using a pH meter.
- Standard: Typically, the pH should be between 6.0–8.0.
5. Impurity Testing
(1) Nucleic Acids
- Standard: Absorbance at 260nm ≤ 0.5 (to ensure minimal nucleic acid residues).
(2) Protein
- Method: Coomassie Brilliant Blue assay.
- Standard: Protein content ≤ 0.1%.
(3) Chlorides
- Method: Silver nitrate titration.
- Standard: Chloride ion content ≤ 0.5%.
(4) Heavy Metals (e.g., Lead, Arsenic, Iron)
- Method: Atomic absorption spectroscopy or colorimetry.
- Standard: Must comply with pharmacopoeia or industry limits (e.g., As ≤ 1ppm, Pb ≤ 5ppm).
6. Microbial Limit Control
Sodium hyaluronate requires strict microbial testing, including:
- Total Aerobic Microbial Count (TAMC): ≤ 100 CFU/g
- Mold & Yeast: ≤ 10 CFU/g
- Pathogens (e.g., Staphylococcus aureus, Pseudomonas aeruginosa): Must be absent.
7. Content Assay
HA content is determined by measuring its basic structural unit—glucuronic acid.
- Method: UV spectrophotometry (530nm wavelength).
- Standard: Typically, content should be ≥ 95%.
SCC’s High Standards for Sodium Hyaluronate Powder
At Stanford Chemicals Company (SCC), we maintain stringent quality control for sodium hyaluronate powder, ensuring compliance with non-GMO, animal-free, kosher, and vegan standards. SCC’s HA is widely used in medical uses, skincare, food, and nutraceutical industries, guaranteeing safety, efficacy, and sustainability.
For high-quality sodium hyaluronate, contact us today: Get A Quote.
